Table of Contents
CBSE Class 9 Science Atoms and Molecules – Chapter Notes
Atom and molecules class 9 Notes:- Atoms and molecules class 9 note pdf download, CBSE Chapter-3 atoms and molecules class 9 notes, Chapter-3 revision notes, class 9 science chapter 3 notes pdf download, atoms and molecules class 9 pdf download. NCERT chapter 3 notes science for class 9th.
Here are the revision Notes of Chapter 3 science CBSE in a short time. Study material notes of atoms and molecules. Here you can find the complete chapter 3 atoms and molecules class 9 notes in one place. NCERT Book for Class 9th Science Chapter 3 Atoms And Molecules
Law of chemical combination
Lavoisier and Joseph L. Prost have given two rules for chemical combinations.
Law of Conservation of Mass:-
According to the Law of Conservation of Mass,
“The mass can neither be created nor destroyed during any chemical reaction.”
After the completion of any chemical reaction, the mass of the product is equal to the mass of the reactants.
For example:- Let us assume that two reactants A & B make two products C & D after the reaction.
A + B → C + D
Then, Mass of (A) + Mass of (B) = Mass of (C) + Mass of (D)
This means that there is no loss of mass during the reaction. Now let’s understand it through a reaction.

Law of Constant Proportion:-
“The ratio of the mass of elements in any compound or chemical substance is always constant.”
For Example:-
Whatever reaction occurs for the formation of water, but the ratio of hydrogen and oxygen elements in it is always 1:8.

If we look at the ratio of the mass of H and O before and after the reaction, it will always be 1: 8.
1. Reactant side:- 4 grams of hydrogen reacts with 32 grams of oxygen to form water. So if we calculate the ratio of mass between hydrogen and oxygen, it will be 1: 8.
2. Product side:- Here if we calculate the ratio of the mass of hydrogen and oxygen in the water compound, it will be 2:16 or 1:8.
Dalton’s Atomic Theory
According to Dalton, “Every matter, whether it is an element or a compound, is made up of very small or tiny particles called atoms.” These atoms cannot be broken further into small pieces by any chemical and physical methods.
Postulates of Daltons Atomic Theory
1. Each substance is made up of very small particles called atoms. Therefore, in any chemical reaction, these atoms participate first.
2. Atoms are fundamental or basic particles of matter that cannot be divided into smaller particles.
3. All atoms of an element have the same mass, size, and chemical properties.
4. Atoms of different elements have different mass, size, and chemical properties.
5. Atoms always combine in a ratio of the whole numbers to form any compound or mixtures.
6. The relative number and kinds of atoms are constant in a given compound.
Atoms, Molecule & Ions
Definition Atoms:-
“The atom is the most fundamental particle of any element or substance from which a substance is formed. In any chemical reaction, atoms participate first.”
Since the size of the atom is very small, its size is measured in nanometers.

Atomic Mass:-
“The atomic mass of an element’s atom indicates how many times the atom of that element is heavier than the 1/12 mass of the carbon-12 atom.”
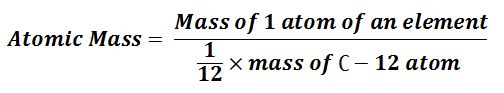
Atomic Mass Unit:- Since the particles of atoms and molecules are very small, a.m.u is used for their measurement.
“One a.m.u (1 u) is defined as it is equal to 1/12th of the mass of the C-12 atom.” It is represented by “amu” or “u”.

Molecule:-
A molecule is formed when two or more (similar or different types of) atoms chemically combine. The molecule remains in an independent state, so it does not participate in any chemical reaction.
For example:-
» The two atoms of the hydrogen element combine to form hydrogen gas(H2).
» Similarly, carbon monoxide(CO) is formed by combining one carbon atom and one oxygen atom.
Molecules of elements:- In this, two or more similar types of atoms of an element combine to form a molecule. Example- H2, O2, Cl2, O3 etc.
Molecules of compounds:-In this, two or more different types of atoms of different elements combine to form one molecule. Example- CO2, NO2, CO, H2O etc.
Atomicity:-
It can define as the total number of atoms present in a molecule or compound is known as atomicity.
Based on the number of atoms present in a molecule classified in the following terms.
➢ Monoatomic – Mono means “single”. Molecules with single or solo atoms are called monoatomic. Example- Noble gases He, Ar, Ne are monoatomic.
➢ Diatomic – A molecule made up of the two (same or different types of) atoms is called a diatomic molecule. Example- O2, H2, N2, CO, HCl etc.
➣ Triatomic – A molecule that consists of the three (same or different types of) atoms is called a triatomic molecule. Example- CO2, H2O, N2O, SO2, O3 etc.
Molecular Mass
The molecular mass is equal to the sum of the mass of each atom present in that molecule.
For example:- Molecular mass of SO2
Mass of Sulphur atom = 32 g
Mass of Oxygen atom = 16 g
So, the molecular mass of SO2 = 32 + (2 × 16) = 64 g
Ions
When an atom becomes electrically charged due to excess or lack of electrons are called ions. OR The charged atoms are called ions.
For example:- When the sodium atom gives up one electron, the sodium is converted into an ion. Similarly, when a chlorine atom gains an electron becomes an ion.

Types of Ions
Since an atom is neutral, it becomes charged when the atom exchanges electrons. Depending on the exchange of electrons, they are of two types.
➢ Cations Ions (Positive):- When the number of protons in an atom exceeds the number of electrons, they are called Cations.
OR
When an atom releases electrons from its outer shell, a positive charge comes on it, which is called a cation. For Example:- ![]()
➢ Anions Ions (Positive):- When the number of electrons in an atom exceeds the number of protons, they are called Anions.
OR
When an atom gains electrons in its outer shell, a negative charge comes on it, which is called an anion. For Example:- ![]()
Formula Unit Mass
The Formula Unit Mass is used to determine the mass of compounds formed from ions. The method of finding the mass of ionic compounds is similar to the method of molecular mass.
OR
It is the sum of the atomic masses of all the atoms present in ionic compounds.
For example:- The chemical formula of salt is NaCl, which is consists of Sodium and Chlorine ions. So, the Formula Unit Mass of NaCl will be;
Atomic mass of Na= 23 g
Atomic mass of Cl= 35.5 g
Formula Unit Mass of NaCl = 23 + 35.5 = 58 g
Chemical Formula
Chemical formulas are a group of symbols that are used to express the molecule of a substance or compound in a concise form.
OR
“The chemical formula is a symbolic representation of any compound.”
Rules for writing Chemical Formula
There are some basic rules to know how to write chemical formulas.
1. If a compound is made of metal and non-metal, then in the chemical formula, metal is written first and non-metal is written later.
For example:-

2. In the case when a compound contains polyatomic ions, the ions are represented with the help of brackets. For a single polyatomic ion, the bracket is not necessary. An example of a single polyatomic ion is NaOH.
For example:-
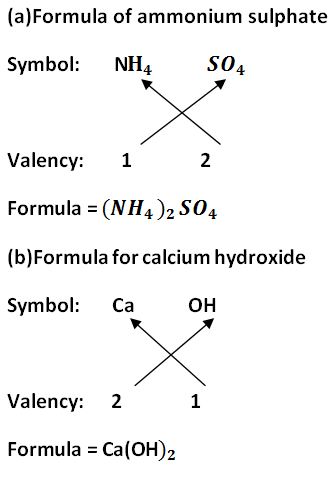 3. The valencies or charges on the ions must be balanced.
3. The valencies or charges on the ions must be balanced.
For example:- ![]()
Mole Concept
“The number of particles present in one mole of a substance is equal to the number of atoms present in exactly 12 grams of carbon-12.”
OR
The quantity of a substance that contains ![]() particles is called 1 mole. Therefore, the number of particles in any substance is fixed no matter what the substance is.
particles is called 1 mole. Therefore, the number of particles in any substance is fixed no matter what the substance is.
So, 1 Mole = ![]() (Atoms, molecules, particles)
(Atoms, molecules, particles)
Avogadro’s Number
The total number of particles present in one mole of any substance is called Avagadro’s Number.
It is represented as ![]()
Formulas:-
Chapter 1- Matter in Our Surroundings Class 9 Notes Science
Chapter 2- Is matter around us pure notes
